Links:
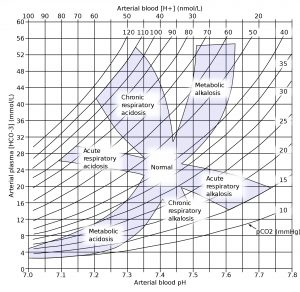
Understanding Acid-Base Disorders
Normal pH
- Important to keep metabolic pathways in their ionized state
- Optimal environment for protein function
Notes on assessment:
- Before obtaining ABG, FiO2 should be stable for 15 min to allow PaO2 and PaCO2 to equilibrate
- Check temperature: measured PaO2 and PaCO2 increase as blood is warmed (O2 dissociation curve shifts right, and solubility of gases decrease)
- Sample should be analyzed immediately or placed on ice
- PaCO2 may increase if sample left at room temp, or if generated by metabolically active leukocytes, red cells
- PaO2 may decrease if consumption in vitro in setting of marked leukocytosis or thrombocytosis
- O2 may diffuse through plastic tube (glass is preferred)
- Normal VBG pH 7.30 – 7.35, Normal PaO2 40 – 44, Normal PaCO2 45
- Expect exaggerated discordance in arterial to venous pH in heart failure
- Acidemia
- Decreased sarcoplasmic Ca2+ release, decreased mitochondrial respiration, decreased enzyme activity
- CNS depression
- Myocardial depression with reduced responsiveness to catecholamines
- Sympathetic overdrive
- Peripheral vasodilitation, Pulmonary vasoconstriction
- Alkalemia
- Decreased ionized Ca2+
- Paresthesias, tetany, seizures, arrhythmias
- Decreased ionized Ca2+
- Decreased sarcoplasmic Ca2+ release, decreased mitochondrial respiration, decreased enzyme activity
Base excess = the amount of strong acid that must be added to 1L fully oxygenated blood to return the pH to 7.40, at pCO2 40 and temp 37
= HCO3– + 10(pH – 7.40) – 24
Bicarb is the predominant base. Negative base excess (base deficit) means that bicarb is depleted
Calculation cannot tell you whether pathologic or compensatory
Bicarbonate: 8.4% (1 mEq/mL) NaHCO3 50 mL = 1 amp (so 3 amps in 1L of D5W gets you 150mEq of NaHCO3)
Image care of https://commons.wikimedia.org